Eliminate media breaks and sign with legal validity using Digital Signatures
Delayed approvals, paper-based processes, and growing compliance requirements slow many companies down. Media breaks complicate traceability and jeopardize process quality. Based on the CONTACT Elements integration platform, Digital Signatures offers an end-to-end and legally compliant solution: documents and data objects can be reviewed, approved, and digitally signed. The solution increases the speed of your approval procedures and ensures secure workflows across departments, locations, and company boundaries.
Your benefits
- Accelerate processes and increase process quality
- Meet regulatory requirements
- Ensure legal validity and security
- Simplify complex workflows and eliminate media breaks
- Sign documents and data objects
- Effectively prevent manipulation and fraud

Accelerate processes and enhance quality

Whether internal review and approval processes or external procedures, such as contract signing: Digital Signatures, based on CONTACT Elements, enables smooth and legally compliant operations in any process environment. Your company significantly reduces paper usage and accelerates processes. Workflows make your operations fully traceable and enhance their quality. Supplementing your approval workflows with digital signature tasks is therefore the decisive step to completely ensure legal enforceability while simultaneously elevating process efficiency and quality to a new level.
Accelerate processes and enhance quality
Whether internal review and approval processes or external procedures, such as contract signing: Digital Signatures, based on CONTACT Elements, enables smooth and legally compliant operations in any process environment. Your company significantly reduces paper usage and accelerates processes. Workflows make your operations fully traceable and enhance their quality. Supplementing your approval workflows with digital signature tasks is therefore the decisive step to completely ensure legal enforceability while simultaneously elevating process efficiency and quality to a new level.

Whether internal review and approval processes or external procedures, such as contract signing: Digital Signatures, based on CONTACT Elements, enables smooth and legally compliant operations in any process environment. Your company significantly reduces paper usage and accelerates processes. Workflows make your operations fully traceable and enhance their quality. Supplementing your approval workflows with digital signature tasks is therefore the decisive step to completely ensure legal enforceability while simultaneously elevating process efficiency and quality to a new level.
Meet regulatory requirements
Increasing compliance regulations demand precise documentation and legally compliant signatures. With CONTACT Elements Digital Signatures, you fulfil the requirements of FDA standard 21 CFR Part 11 for electronic records and signatures, as well as EU Regulation No. 910/2014 (eIDAS), which governs electronic identification and trust services. Already today, there are several established standards that require or at least encourage digital signatures. For example, the automotive industry recommends them for data transfer via the secure OFTP2 protocol for Electronic Data Interchange (EDI).
Meet regulatory requirements
Increasing compliance regulations demand precise documentation and legally compliant signatures. With CONTACT Elements Digital Signatures, you fulfil the requirements of FDA standard 21 CFR Part 11 for electronic records and signatures, as well as EU Regulation No. 910/2014 (eIDAS), which governs electronic identification and trust services. Already today, there are several established standards that require or at least encourage digital signatures. For example, the automotive industry recommends them for data transfer via the secure OFTP2 protocol for Electronic Data Interchange (EDI).
Increasing compliance regulations demand precise documentation and legally compliant signatures. With CONTACT Elements Digital Signatures, you fulfil the requirements of FDA standard 21 CFR Part 11 for electronic records and signatures, as well as EU Regulation No. 910/2014 (eIDAS), which governs electronic identification and trust services. Already today, there are several established standards that require or at least encourage digital signatures. For example, the automotive industry recommends them for data transfer via the secure OFTP2 protocol for Electronic Data Interchange (EDI).
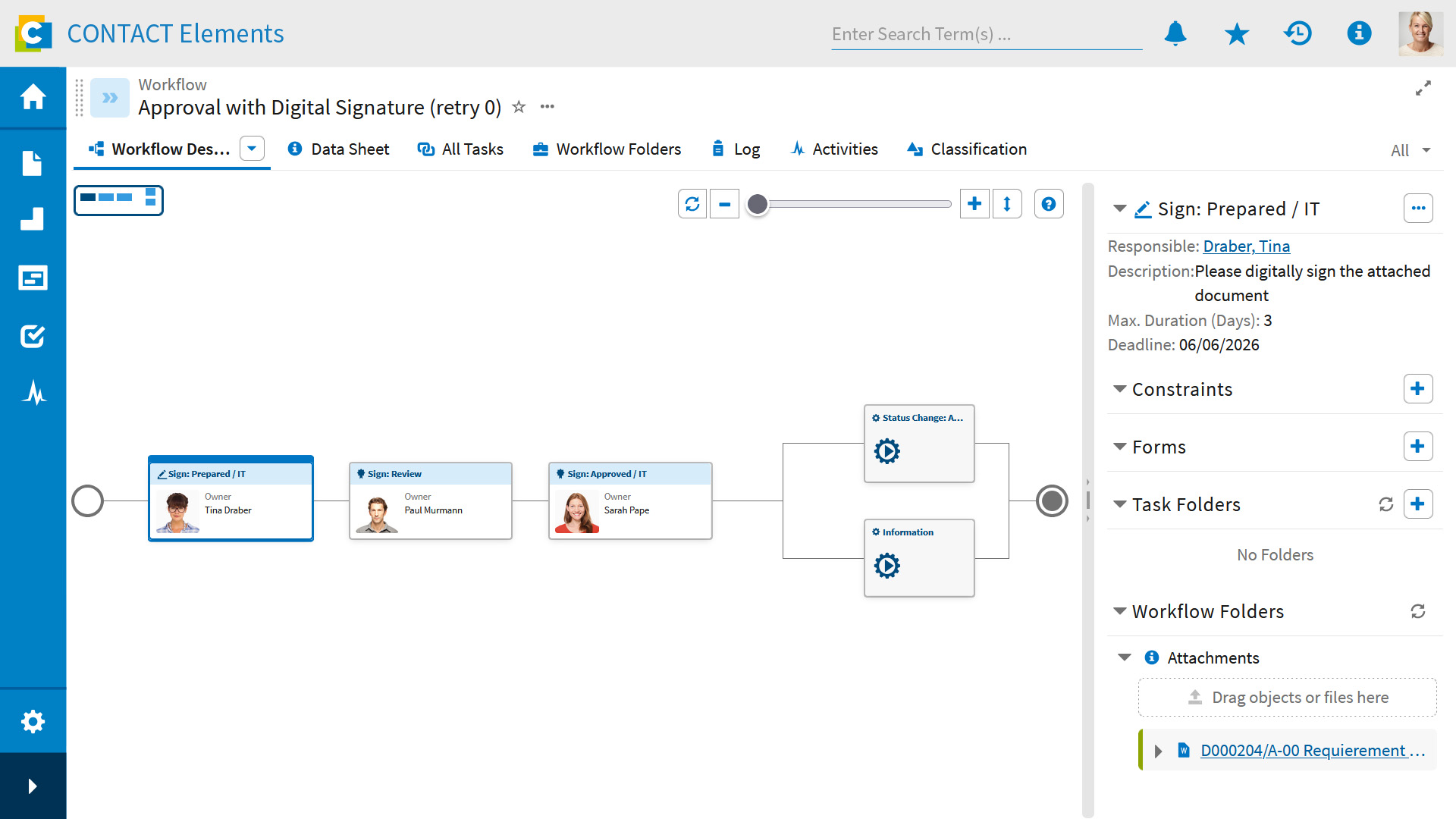
Simplify complex workflows and eliminate media breaks
Digital Signatures, especially when combined with CONTACT Elements as an integration platform, truly unlocks its full potential in multi-stage review and approval processes. You can seamlessly combine predefined workflows with signing tasks. Users see their assigned tasks directly in the Task Manager and can complete them immediately – without media breaks or system changes. This significantly accelerates processing times while simultaneously increasing process security and traceability, particularly for distributed teams.
Simplify complex workflows and eliminate media breaks
Digital Signatures, especially when combined with CONTACT Elements as an integration platform, truly unlocks its full potential in multi-stage review and approval processes. You can seamlessly combine predefined workflows with signing tasks. Users see their assigned tasks directly in the Task Manager and can complete them immediately – without media breaks or system changes. This significantly accelerates processing times while simultaneously increasing process security and traceability, particularly for distributed teams.

Digital Signatures, especially when combined with CONTACT Elements as an integration platform, truly unlocks its full potential in multi-stage review and approval processes. You can seamlessly combine predefined workflows with signing tasks. Users see their assigned tasks directly in the Task Manager and can complete them immediately – without media breaks or system changes. This significantly accelerates processing times while simultaneously increasing process security and traceability, particularly for distributed teams.
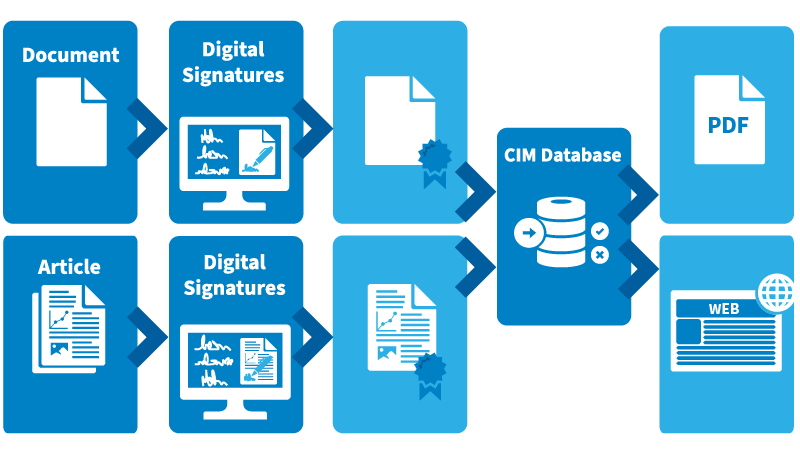
Sign documents and data objects
In addition to documents, you can digitally sign workflows, assemblies, or articles in a legally valid manner – even CAD drawings with automatically populated title blocks. Enable internal stakeholders and external customers or partners to digitally sign requirements or complex product structures. You can also secure parts with their associated bills of material in an approved version using signatures. This way, approvals with signatures can be legally and digitally represented across all process stages. The result is end-to-end traceable, binding, and cost-efficient processes with high protection against manipulation and fraud.
Sign documents and data objects
In addition to documents, you can digitally sign workflows, assemblies, or articles in a legally valid manner – even CAD drawings with automatically populated title blocks. Enable internal stakeholders and external customers or partners to digitally sign requirements or complex product structures. You can also secure parts with their associated bills of material in an approved version using signatures. This way, approvals with signatures can be legally and digitally represented across all process stages. The result is end-to-end traceable, binding, and cost-efficient processes with high protection against manipulation and fraud.

In addition to documents, you can digitally sign workflows, assemblies, or articles in a legally valid manner – even CAD drawings with automatically populated title blocks. Enable internal stakeholders and external customers or partners to digitally sign requirements or complex product structures. You can also secure parts with their associated bills of material in an approved version using signatures. This way, approvals with signatures can be legally and digitally represented across all process stages. The result is end-to-end traceable, binding, and cost-efficient processes with high protection against manipulation and fraud.

Compliant Use of Digital Signatures
A multitude of laws, guidelines and directives regarding consumer protection, product liability, environmental and quality management mean that companies in regulated industries have to meet stringent regulatory requirements – this applies not only to the financial industry but also to the automotive, air transport, energy, health care, life science and pharmaceutical industries. Companies in these industries therefore have to commit considerable resources to ensuring that processes meet these requirements in a traceable manner and providing appropriate certification, as well as guaranteeing that contracts are legally valid. After a brief definition of the terminology used and a description of how digital signatures work, this white paper discusses the legal specifics of digital signatures. This is followed by an introduction to two directives from regulated industries that stipulate the use of digital signatures, namely the OFTP2 transfer protocol and 21 CFR Part11. Finally, this white paper describes the possibility of integrating digital signatures in PLM processes and the resulting benefits for companies.
Related Elements
Organize and utilize know-how enterprise wide
Make all important documents available on every workstation
Save valuable time when searching for documents
Protect valuable know-how
Speed up test and release processes
Comply with regulatory requirements more easily
Control tasks, standardize and accelerate processes
Map and standardize recurring processes as process templates
Speed up processes and ensure compliance
Direct integration in task and project management
Regulate data access, protect know-how
Implement key requirements for know-how protection reliably throughout the entire company
Take advantage of the possibilities offered by a powerful and dynamic rights system
Regulate access to project and product data along your processes based on roles
Ensure validity control with reliable version management
Further information
Would you like to find out more about this topic? Choose one of the following information offers.

